Abstract
Introduction: Sickle cell disease (SCD) is a genetic disorder where deoxygenation produces polymerization of mutated hemoglobin S and triggers red cell distortion (sickling), hemolysis, vaso-occlusion and inflammation. Injury from SCD accumulates over time causing significant end-organ damage and ischemic tissue injury, leading to fatigue, pain and other clinical complications that are under-recognized, under-treated, and associated with early death. Guideline-recommended care includes the use of blood transfusions and routine use of hydroxyurea (HU) to reduce the incidence of vaso-occlusive crisis, acute chest syndrome, and reduce mortality. In addition, individuals with SCD benefit from access to hematologists for on-going treatment. Children with SCD in the United States (US) often receive comprehensive care through multidisciplinary SCD clinics. Adults, however, have no such comprehensive care model, and availability of physicians specializing in SCD and of insurance coverage may complicate their access to care. Previous research has examined challenges faced by individuals with SCD as they transition from pediatric to adult care. This study adds to current knowledge by describing the use of key treatments and services in a large cohort of SCD patients in the US.
Methods: The Truven MarketScan® Commercial and Medicaid administrative claims databases were used to identify patients with SCD (1 inpatient or 2 outpatient claims with ICD-9 282.6x, 282.41, and/or 282.42) recorded in any calendar year from 2009 to 2014; 1 cohort was constructed for each year, and patients may have been diagnosed previously. Patients aged ≥ 9 months at first indication of SCD were included. In each cohort, patients were required to be enrolled in medical and pharmacy benefits for the calendar year in which they were identified. The final cohorts were restricted to patients with 12 months continuous medical and pharmacy benefits coverage prior to their first recorded indication of SCD in the cohort year. Prior year utilization of healthcare resources (visits to hematologist/oncologist or emergency department [ED], and inpatient [IP] admissions), and HU was determined for each cohort. Oncologists were grouped with hematologists as they generally have a secondary specialty (not captured in database) of hematology. Results are reported as ranges across the annual cohorts, stratified by payer (Commercial or Medicaid) and age group (<6, 6-11, 12-17, 18-30, 31-44, 45+).
Results: There were 2,619-3,285 Commercial and 4,807-7,007 Medicaid SCD patients identified in each of the annual cohorts. The mean age was 27 years in the Commercial cohorts, and over 50% of patients were female. The mean age was 18 in the Medicaid cohorts and about 50% were female.
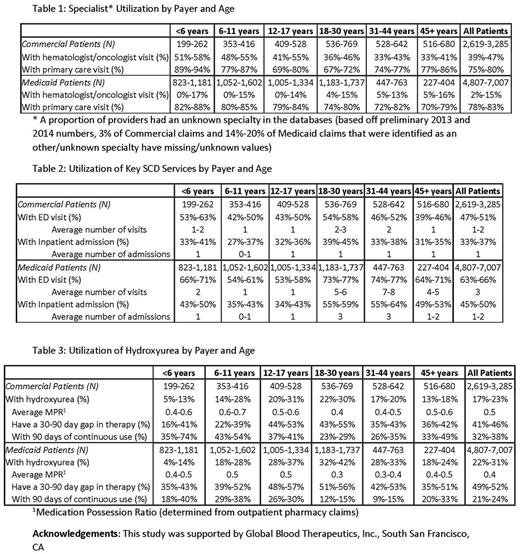
The proportion of SCD patients who saw a hematologist/oncologist in the prior year was higher in the Commercial cohorts (39%-47%) compared to the Medicaid cohorts (2%-15%) across all age groups. In Commercial patients, the proportion of adults (age >18) with a hematologist/oncologist visit was substantially lower than the proportion of children with such visits (Table 1). The proportion of patients with a primary care visits was highest among the <6 age group and lowest among adults (Table 1).
The average number of ED visits was higher in Medicaid cohorts (≥3) compared to Commercial cohorts (≥1) in adults. A higher proportion of patients in the Medicaid cohorts had IP admissions compared to patients in the Commercial cohorts. The differences were more pronounced in the adult cohorts than in the pediatric cohorts for both payers (Table 2).
Only 22%-31% of the SCD patients in both payers had at least one outpatient HU claim. Patients with at least 90 days of continuous HU use was lowest for age group 18-30 years in Medicaid (12%-15%) and Commercial (23%-29%) cohorts (Table 3).
Conclusions: Use of hematology/oncology care was strikingly low among Medicaid SCD patients. This finding suggests that SCD patients in Medicaid plans may have less access to hematologists/oncologists than patients with commercial insurance. This limited use of specialty care may reduce the preventative care Medicaid patients receive. The higher ED and inpatient use and lower HU compliance in the Medicaid population may be indicative of greater severity and/or unmet need. This data highlights the importance of ongoing initiatives such as the ASH SCD Coalition to increase access to care in the US.
Dampier: Pfizer, Lilly, Novartis, Sancilio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kanter: American Society of Hematology (Sickle Cell Disease Guideline Panel): Membership on an entity's Board of Directors or advisory committees; GBT: Research Funding; Sancillo: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; NHLBI (sickle cell disease research advisory committee): Membership on an entity's Board of Directors or advisory committees, Research Funding; Apopharma: Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; MUSC: Other: The site PI for sponsored research conducted at MUSC who receives funds from: Novartis, bluebird bio, GBT, Sancillo, Apopharma, Pfizer. Howard: Global Blood Therapeutics: Employment, Equity Ownership. Agodoa: Global Blood Therapeutics, Inc.: Employment, Equity Ownership. Wade: Truven Health Analytics, an IBM company which received project funding from Global Blood Therapeutics: Consultancy. Noxon: Truven Health Analytics, an IBM company which received project funding from Global Blood Therapeutics: Employment. Ballas: Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal